MERCURY VAPOR IN WORKPLACE ATMOSPHERES
| Method Number: | ID-140 |
| Matrix: | Air |
| OSHA Permissible Exposure
Limits Mercury Vapor (Final Rule Limit): |
0.05 mg/m3 (TWA) |
| Mercury (Transitional Limit): | 0.1 mg/m3 as total mercury (TWA) |
| Collection Device: | A passive or an active sampling device are available. Both devices use HydrarR or hopcalite as the solid sorbent. |
| Recommended Sampling
Rate Passive Dosimeter: |
0.020 L/min (@ 20 °C and 101 kPa) |
| Active Sampler: | 0.20 L/min |
| Recommended Air Volume
Range Passive Dosimeter: |
9.6 L |
| Active Sampler: | 3 to 100 L |
| Analytical Procedure: | The sorbent is digested using nitric acid and
hydrocloric acid. The mercury in the sample is reduced to elemental
mercury using stannous chloride and analyzed using a cold
|
| Detection Limit | |
| Qualitative Passive Dosimeter: Active Sampler: |
0.002 mg/m3 for a
0.00067 mg/m3 for a |
| Quantitative Passive Dosimeter: Active Sampler: |
0.004 mg/m3 for a
0.0013 mg/m3 for a |
| Precision and
Accuracy Passive Dosimeter Validation Range: |
0.061 to 0.20 mg/m3 |
| CVT(pooled) | 0.039 |
| Bias | +0.008 |
| Overall Error | ±8.6% |
| Method Classification: | Validated Method |
| Date (Date Revised): | 1987 (June, 1991) |
Commercial manufacturers and products mentioned in this
method are for
descriptive use only and do not constitute endorsements
by
Similar products from other sources can be
substituted.
Division of Physical Measurements and Inorganic
Analyses
OSHA Technical Center
Salt Lake City, Utah
1. Introduction
This method describes the collection of airborne elemental mercury in a
passive dosimeter or active sampling device and subsequent analysis using
a cold
-
The mercury vapor and hence the entire sample was immediately lost into the surrounding atmosphere
-
The method was imprecise at lower sample loadings (8.4.)
-
The analytical technique was somewhat tedious
1.1. Principle
The mercury dosimeter samples the workplace atmosphere by controlled diffusion into the badge while the active sampler uses a calibrated sampling pump. The mercury vapor entering either passive or active device is collected on a solid sorbent (HydrarR or hopcalite) which has an irreversible affinity for mercury (8.1., 8.2.). After sample collection the sorbent is initially dissolved with concentrated nitric acid and then hydrochloric acid. Stannous chloride is added to an aliquot of the sample to generate mercury vapor. This vapor is then driven into an absorption cell of a flameless atomic absorption spectrophotometer for analysis.
1.2. History
Previously, mercury samples were collected on
Hopcalite solid sorbent (8.5.) was substituted in place of the iodine-impregnated charcoal for mercury vapor sampling. Previously, hopcalite had been used in respirator cartridges for carbon monoxide and consisted of oxides of copper, manganese, cobalt, and silver (8.6.). Analysis of recent batches of hopcalite used for mercury collection indicate the composition was mainly oxides of manganese and copper.
HydrarR has been used as a substitute for collecting mercury vapor and is very similar in composition to hopcalite. A ceramic material, insoluble in nitric and hydrochloric acid, is present in the HydrarR but not in the hopcalite.
1.3. Advantages and Disadvantages
1.3.1. These sampling and analytical techniques have adequate sensitivity for measuring workplace atmospheric concentrations of elemental mercury.
1.3.2. The passive dosimeter used for collection of mercury vapor is small, lightweight, and requires no sampling pumps. Also, the dosimeter housing is reusable; therefore, cost per measurement is kept to a minimum.
1.3.3. The collected mercury sample is stable for at least 30 days.
1.3.4. Sample preparation for analysis involves simple procedures.
1.3.5. Either sampling device can be analyzed in any laboratory
equipped with a
1.3.6. A disadvantage with the passive dosimeter is particulate compounds cannot be collected with the device. A separate sampling pump and collection media should be used for particulate collection.
1.3.7. Another disadvantage with the dosimeter is sample rate dependence on face velocity. The dosimeter should not be used in areas where the air velocity is greater than 229 m/min (750 ft/min) since erratic increases in sampling rate may occur.
1.3.8. A disadvantage with the active device is the dependence on a calibrated pump to take the sample.
1.4. Toxic Effects (This section is for information only and should not be taken as a basis for OSHA policy.)
Exposure to elemental mercury vapor can occur via the respiratory tract and skin. Possible symptoms from an acute exposure include severe nausea, vomiting, abdominal pain, bloody diarrhea, kidney damage, and death. These symptoms usually present themselves within 10 days of exposure. Potential symptoms from a chronic exposure include inflammation of the mouth and gums, excessive salivation, loosening of the teeth, kidney damage, muscle tremors, jerky gait, spasms of the extremities, personality changes, depression, irritability, and nervousness (8.7., 8.8.).
1.5. Workplace Exposure
Occupations with potential exposure to mercury and its compounds are listed (8.8.):
| amalgam makers | fur processors |
| bactericide makers | gold extractors |
| barometer makers | histology technicians |
| battery makers, mercury | ink makers |
| boiler makers | insectiside makers |
| bronzers | investment casting workers |
| calibration instrument makers | jewelers |
| cap loaders, percussion | laboratory workers, chemical |
| carbon brush makers | lampmakers, fluorescent |
| caustic soda makers | manometer makers |
| ceramic workers | mercury workers |
| chlorine makers | miners, mercury |
| dental amalgam makers | neon light makers |
| dentists | paint makers |
| direct current meter workers | paper makers |
| disinfectant makers | percussion cap makers |
| disinfectors | pesticide workers |
| drug makers | photographers |
| dye makers | pressure gage makers |
| electric apparatus makers | refiners, mercury |
| electroplaters | seed handlers |
| embalmers | silver extractors |
| explosive makers | switch makers, mercury |
| farmers | tannery workers |
| fingerprint detectors | taxidermists |
| fireworks makers | textile printers |
| fungicide makers | thermometer makers |
| fur preservers | wood preservative workers |
1.6. Properties (8.7., 8.8.)
Elemental mercury (CAS No.
| Atomic Number | 80 |
| Atomic Symbol | Hg |
| Atomic Weight | 200.61 |
| Freezing Point | -38.87 °C |
| Boiling Point | 356.90 °C |
| Density | 13.546 g/mL (20 °C) |
| Synonyms | Quicksilver, Hydrargyrum |
The high vapor pressure of mercury at normal temperatures combined with the potential toxicity makes good control measures necessary to avoid exposure. Also, the concentration of mercury vapor in the air rapidly increases as the temperature increases. To illustrate, listed below are vapor pressures of mercury, and mercury concentrations of air after saturation with mercury vapor at different temperatures:
Vapor
|
| ||||
| Temperature | Vapor Pressure | Mercury Concentration | ||
| °C | °F | (torr) | (µg/m3) | |
|
| ||||
| 0 | 32.0 | 0.000185 | 2,180 | |
| 10 | 50.0 | 0.000490 | 5,880 | |
| 20 | 68.0 | 0.001201 | 13,200 | |
| 24 | 75.2 | 0.001691 | 18,300 | |
| 28 | 82.4 | 0.002359 | 25,200 | |
| 30 | 86.0 | 0.002777 | 29,500 | |
| 32 | 89.6 | 0.003261 | 34,400 | |
| 36 | 96.8 | 0.004471 | 46,600 | |
| 40 | 104.0 | 0.006079 | 62,600 | |
|
| ||||
2. Range
- 2.1. The qualitative and quantitative detection limits for the
analytical procedure are 0.01 µg and 0.02 µg mercury, respectively
(8.9.).
2.2. Working Range
The range of the analytical procedure has been determined to be 0.1 to 2 µg mercury. Using the analytical conditions specified, a nonlinear response was noted above 2 µg.
3. Method Performance
3.1. The SKC HydrarR gas monitoring dosimeter badge for mercury (SKC Inc., Eighty Four, PA) was evaluated at 80% RH and 25 °C over the range of 0.061 to 0.203 mg/m3 using a dynamic generation system (8.2.). The pooled coefficient of variation (CVT) for badge samples taken in this concentration range was 0.039. The average recovery was 100.8% and the overall error was ±8.6%.
In a separate study, active samplers were spiked with mercury in the range of 1 to 2.5 µg. The mean recovery of these 125 quality control samples was 96.9% with a CV1 of 0.106 (8.10.).
3.2. In storage stability studies, the mean recoveries of HydrarR samples analyzed 5, 14, and 30 days after collection were within ±10% of the known generated concentration (8.2.).
3.3. The HydrarR active sampling device was compared using linear regression statistics to the dosimeter in a field study (8.11.). The dosimeter results agreed well with the active sampler and are summarized below (Note: A correlation coefficient and slope = 1 would indicate ideal agreement):
| Number of paired samples | (N) | = | 26 |
| Concentration Range | = | 0.01 to 0.7 mg/m3 | |
| Correlation coefficient | (r) | = | 0.985 |
| Intercept | (a) | = | 0.017 |
| Slope | (b) | = | 0.960 |
| Standard deviation of the slope | (Sb) | = | 0.038 |
4. Interferences
4.1. Sampling:
Particulate mercury compounds are a positive interference; however, the badge does not sample particulates and the glass wool of the active sampler prevents particulate from entering the sorbent. Chlorine in the sampled air does not interfere when using HydrarR or hopcalite sorbent. The chlorine does react with available mercury vapor in the air to presumably form mercuric chloride (8.12.). Workplaces containing both chlorine and mercury should be sampled for both mercury vapor and particulate.
4.2. Analysis:
Organic-free deionized water should be used during sample and
standard preparation. Any compound with the same absorbance wavelength
as mercury (253.7 nm) can be a positive interference. Some volatile
organic compounds (i.e. benzene, toluene, acetone, carbon
tetrachloride) absorb at this wavelength and are considered analytical
interferences. They occur as contaminants in the reagents used during
sample preparation. These compounds are not expected to be retained on
HydrarR or hopcalite during sample
collection. Analytical interferences are rendered insignificant by
using
Increasing the concentration of nitric acid in the samples or standards appears to produce an elevated background signal. The nitric acid concentration in the samples and standards should not be greater than 10%.
5. Sampling
[Note: A prefilter assembly, consisting of a
-
The air velocity of the sampling site is greater than 229 m/min (750 ft/min)
-
The operation being sampled is characterized by extremely poor hygienic practices and splashing of mercury on the badge may occur
-
Determination of compliance to the Transitional Permissible Exposure Limit (PEL) to total mercury is necessary and mercury particulate appears to be present in the workplace atmosphere
-
particulate mercury compounds may present a problem during sampling or
-
the hopcalite or HydrarR contained in the active sampling tube has migrated to the glass wool plug.
5.1. Equipment
Either tubes or dosimeters can be used to collect mercury vapor. The dosimeter should not be used when:
The tube can be used to determine compliance with the Transitional PEL of 0.1 mg/m3 (TWA) as total mercury (vapor + particulate). The badge can only collect mercury vapor. For Ceiling exposures to particulate mercury, or for wipe and bulk sampling and analysis consult reference 8.14. for further information.
5.1.1. PASSIVE DOSIMETER:
Gas monitoring dosimeter badge and pouch containing a
HydrarR capsule [badge - cat. no.
5.1.2. ACTIVE SAMPLER:
HydrarR or hopcalite sampling tubes
(cat. no.
Note: Before use, the active sampling tubes must be examined for movement of the the solid sorbent into the glass wool. See Section 5.3.1. for further details.
5.1.3. Sampling pumps capable of sampling at 0.2 liters per minute (L/min).
5.1.4. Assorted flexible tubing.
5.1.5. Stopwatch and bubble tube or meter for pump calibration.
5.2. Sampling Procedure - PASSIVE DOSIMETER
5.2.1. Assemble the components of the mercury monitoring badge according to manufacturer instructions (8.1.).
Note: A foam insert must be placed in the Model
5.2.2. Record the sampling start time, sampling site temperature, and atmospheric pressure. Remove the protective cap and then place the dosimeter in the breathing zone of the employee. The suggested sampling time for the dosimeter is 8 h.
5.2.3. Immediately after sampling, carefully remove the sorbent capsule from the dosimeter and place it in the sorbent pouch. Fold the pouch top twice and press it flat to seal the capsule inside the pouch. Record the sampling stop time, final temperature, and atmospheric pressure. Calculate and record the total sampling time, average temperature, and pressure.
5.3. Sampling Procedure - ACTIVE SAMPLER
5.3.1. Calibrate each personal sampling pump with an active
sampler
Note: A prefilter assembly consisting of a
Before use, the active sampling tubes must be examined for
movement of the solid sorbent into the glass wool. Certain lots of
HydrarR or hopcalite have been noted as
being very friable or having a sorbent
5.3.2. Connect a sampling tube (or sampling assembly) to a calibrated pump using flexible tubing. If a prefilter is used, connect it to the sampling tube with a minimum amount of Tygon tubing. Connect the other end of the sampling tube to the pump. Place the sampling tube (or assembly) in the breathing zone and the pump in an appropriate position on the employee.
5.3.3. Use an air volume in the range of 3 to 100 L to collect the mercury in the workplace air. Record the total volume.
5.3.4. Replace the plastic end caps on the active sampler after sampling is completed.
5.4. Sample Shipment
5.4.1. Securely wrap each sorbent pouch or active sampling tube
5.4.2. Submit at least one blank sample with each set of samples. The blank sample should be handled in the same manner as the other samples except that an air sample is not taken.
5.4.3. Request the laboratory to analyze the samples for mercury. Submit any pertinent sampling information to the lab. Record if a prefilter assembly was used.
5.4.4. Ship the sealed pouches and used dosimeter housings, or active sampling tubes to the laboratory in appropriate containers as soon as possible. The filter/cassette assembly can also be submitted for mercury particulate analysis; however, sampling periods may be longer than reflected in exposure regulations. The PEL for mercury particulate is a Ceiling (8.14.) and the vapor is a TWA PEL.
6. Analysis
-
If a prefilter was not used during sampling, place the glass wool and sorbent into separate
50-mL volumetric flasks. -
If a prefilter was used and the glass wool appears to contain hopcalite or Hydrar, the glass wool can be analyzed along with the sorbent. Carefully transfer the glass wool and sorbent to a
50-mL volumetric flask without losing any of the particles. - start the chart recorder,
- set the spectrophotometer absorbance reading to zero,
- wait until the baseline stops drifting,
- set the reading to zero again.
6.1. Safety Precautions
6.1.1. Wear safety glasses, labcoat, and gloves at all times.
6.1.2. Handle acid solutions with care. Avoid direct contact of acids with work area surfaces, eyes, skin, and clothes. Flush acid solutions which contact the skin or eyes with copious amounts of cold water.
6.1.3. Prepare solutions containing hydrochloric acid in an
exhaust hood and store in
6.1.4. Keep B.O.D. bottles containing stannous chloride/hydrochloric acid solutions capped when not in use to prevent inhalation of noxious vapors.
6.1.5. Exercise care when using laboratory glassware. Do not use chipped pipets, volumetric flasks, beakers or any glassware with sharp edges exposed.
6.1.6. Never pipet by mouth.
6.1.7. When scoring the glass of active samplers to remove the
sorbent before analysis, score with care. Apply only enough pressure
to scratch a clean mark on the glass. Use a paper towel or cloth to
support the opposite side while scoring. Moisten the mark with DI
H2O and wrap the tube in cloth before
breaking. If the tube does not break easily,
6.1.8. Always purge the mercury from the
6.1.9. Occasionally monitor the
6.2. Equipment - Cold Vapor Analysis
(Note: Specific equipment is listed for illustration only)
6.2.1. Atomic absorption spectrophotometer (model 503,
6.2.2. Mercury hollow cathode lamp or electrodeless discharge lamp and power supply.
6.2.3. Biological Oxygen Demand (B.O.D.) bottles, borosilicate glass, 300 mL.
6.2.4. Peristaltic pump, 1.6 to 200 mL range, and controller,
6.2.5. Quartz absorption cell,
6.2.6. Heating tape.
6.2.7. Variable transformer
6.2.8. Tygon peristaltic pump tubing (part no.
6.2.9. Aerator (part no.
6.2.10. Chart recorder.
6.2.11. Desiccant (Drierite, W.A. Hammond Drierite Co., Xenia, OH).
6.2.12. Volumetric flasks, volumetric pipets, beakers, and other laboratory glassware.
6.2.13. Automatic pipets, adjustable, 0.1 to 5.0 mL range (models
6.2.14. Glass tube scorer, or needle, 21 to 25 gauge - for removing metal screens in dosimeters or glass wool from tubes. A piece of bent wire can also be used.
6.2.15. Exhaust vent.
6.3. Reagents - All reagents should be at least reagent grade.
Stannous chloride, (SnCl2)
6.3.1. Deionized water (DI H2O), organic-free.
6.3.2. Hydrochloric acid (HCl), concentrated (36.5 to 38%), with a mercury concentration less than 0.005 ppm.
6.3.3. Mercury standard stock solution, 1,000 µg/mL: Use a commercially available certified standard or, alternatively, dissolve 1.0798 g of dry mercuric oxide (HgO) in 50 mL of 1:1 hydrochloric acid and then dilute to 1 L with DI H2O. Store this reagent in a dark environment, preferably in an amber colored container.
6.3.4. Nitric acid (HNO3), concentrated (69 to 71%), with a mercury concentration less than 0.005 ppm.
6.3.5. Nitric acid, 1:1: Carefully add equal portions of concentrated HNO3 and DI H2O.
6.3.6. Nitric acid, 10%: Carefully add 100 mL concentrated HNO3 to 900 mL DI H2O.
6.3.7. Stannous chloride (SnCl2) solution, 10%: Dissolve 20 g SnCl2 in 100 mL concentrated HCl. Slowly and carefully pour this solution into 100 mL DI H2O and then mix well. Transfer and store the final solution in a capped B.O.D. bottle to prevent oxidation. Prepare this solution before each new analysis.
6.4. Glassware Preparation
6.4.1. Clean the B.O.D. bottles and stoppers with 1:1 HNO3 and thoroughly rinse with DI H2O prior to use.
6.4.2. Rinse all other glassware with 10% nitric acid and then
with DI H2O prior to use. Air dry all
6.5. Standard Preparation
6.5.1. Prepare a 1 µg/mL mercury standard by making appropriate
6.5.2. Prepare working mercury standards (ranging from 0.1 to 2.0
µg) and reagent blanks immediately prior to use. A few
standards at each concentration should be made. Add an appropriate
aliquot of the 1 µg/mL standard to a clean B.O.D. bottle containing
enough 10% HNO3 to bring the total volume
to 100 mL. A suggested dilution scheme is given:
|
| ||
| Mercury Standard (µg) | Aliquot (mL)* | Final Volume (mL) |
|
| ||
| Reagent Blank | 0 | 100 |
| 0.1 | 0.1 | 100 |
| 0.2 | 0.2 | 100 |
| 0.5 | 0.5 | 100 |
| 1.0 | 1.0 | 100 |
| 1.5 | 1.5 | 100 |
| 2.0 | 2.0 | 100 |
| * Aliquot taken from 1 µg/mL standard prepared in Section 6.5.1. | ||
|
| ||
6.6. Sample Preparation [Note: A hooked needle or piece of fine wire is useful to remove the dosimeter screen or glass wool (active sampler) and the sorbent particles.]
6.6.1. DOSIMETER
Open each sample pouch and remove the sorbent capsule. Carefully
remove the screen from the top of the capsule without losing any
sorbent. Carefully pour the sorbent into a clean, dry
6.6.2. ACTIVE SAMPLER
Score the tube with a glass tube cutter (also see Section 6.1.7.) and then break open the front section of the tube above the glass wool. An alternative approach to scoring and breaking is to carefully remove the glass wool with a bent wire or needle.
6.6.3. Prefilter
Prepare and analyze any prefilters according to reference 8.14.
6.6.4. Add 2.5 mL of concentrated HNO3 followed by 2.5 mL concentrated HCl to each volumetric flask [Note: To minimize any loss of mercury through a change in oxidation state, the HNO3 is added before the HCl (8.5.)].
6.6.5. Gently swirl the sample occasionally for approximately 1
h. If HydrarR was used to collect the
sample, the dark brown solution will also contain some undissolved
clear to
6.6.6. Carefully dilute to a
6.7. Analysis - Instrument Parameters
6.7.1. Set up the
6.7.2. Wrap the heating tape around the quartz cell and then turn on the variable transformer. The heat setting on the tape should be sufficient to prevent water vapor condensation in the absorption cell.
6.7.3. Place the aerator in a B.O.D. bottle which contains approximately ½ to 1 inch of desiccant. Operate the peristaltic pump for approximately 30 min at full speed to remove any water vapor from the system.
6.7.4. Operate the hollow cathode or electrodeless discharge mercury lamp at the manufacturer's recommended current or power rating.
6.7.5. Use the following settings (Note: The mentioned instrument
settings are for specific models used at the
Atomic Absorption Spectrophotoineter:
| Slit | 0.7 nm |
| Signal | Repeat Mode |
| Function | ABS |
| Mode | ABS |
| Range | UV |
| Wavelength | 253.7 nm |
| Filter | Out |
| EM Chopper | Off |
| Phase | Normal |
Strip Chart Recorder:
| Chart Speed | 5 mm/min |
| Chart Range | 10 mV |
6.7.6. Optimize the ENERGY meter reading at 253.7 nm.
6.7.7. Align the beam of the mercury lamp so it passes directly through the center of the quartz cell windows. This can be accomplished by adjusting the burner height, depth, and angle knobs to give a minimum ABSORBANCE reading.
6.7.8. Operate the peristaltic pump at full speed. Rinse the aerator with DI H2O and insert it into a holder in the exhaust vent.
6.7.9. Perform the following steps to obtain a baseline signal near an absorbance of zero:
6.8. Analysis
6.8.1. Samples: Immediately before analyzing, transfer an appropriate aliquot of the sample solution to a clean B.O.D. bottle containing enough 10% HNO3 solution to bring the total volume to 100 mL. The transfer must be done with a volumetric pipet.
6.8.2. Standards: Immediately before analyzing, prepare standards according to instructions listed in Section 6.5.2.
6.8.3. Deliver 5 mL of the 10% SnCl2 solution with an automatic pipet to a B.O.D. bottle containing a standard, reagent blank, or sample to be analyzed. Immediately place the aerator into the solution with the peristaltic pump operating at full speed.
6.3.4. Record the maximum absorbance reading and label the signal produced on the strip chart.
6.8.5. Stop the pump, remove the B.O.D. bottle from the
6.8.6. If the absorbance reading of a sample is greater than the
highest standard at any time during analysis,
immediately remove the B.O.D. bottle from the
6.8.7. Repeat Sections 6.8.3. through 6.8.5. for each prepared standard, reagent blank, or sample.
6.9. Analytical Recommendations
6.9.1. It is recommended to analyze the reagent blank, lowest,
and highest standard two or three times each to check for
contamination, reproducibility, and sensitivity before starting the
sample analysis. A 2.0-µg mercury standard should give a
6.9.2. It is also recommended to analyze an entire series of standards (including the reagent blank) at the beginning and end of the sample analysis to ensure standard readings are reproducible. As a general guideline, standard readings should be within ±10% throughout the analysis.
6.9.3. A standard near the concentration range of the samples should be analyzed after every four to five samples.
6.9.4. Quality control (QC) samples should be prepared and analyzed using the same matrix and analytical conditions as the samples. If possible, the QC samples should be generated from an independent source.
6.9.5. Approximately 10% of the samples should be reanalyzed.
7. Calculations
- mg/m3 mercury vapor
- mg/m3 total mercury
7.1. Use a least squares regression program to plot a
7.2. Determine the amount (µg) of mercury, A, corresponding to the peak absorbance in each analyzed sample aliquot from this curve.
7.3. Calculate the total amount (µg) of mercury, W, in each sorbent
or glass wool sample:
| W = | (A) (sample volume, mL) (DF)
(aliquot, mL) |
Where:
DF = Dilution Factor (if none, DF = 1)
7.4. A blank correction is made for each sample (Note: When using
the reagents and conditions specified, previous blank results have
been less than 1 µg). Calculate the concentration of mercury in each
sorbent or glass wool sample:
| mercury mg/m = | W - Wb
air volume, L |
Where:
| Wb | = | Total µg of mercury in the blank sample. |
| Air vol | = | Sampling time × flow rate (for ACTIVE SAMPLERS) |
| (Note: | For PASSIVE DOSIMETERS, the sampling rate is affected by temperature and pressure. To correct for this, use: |
Air vol = ST × 0.020 × (T1/T2)1.5 × (P2/P1)
Where:
| ST | = | Sampling time (min) |
| 0.020 | = | Sampling rate (L/min) at 20 °C and 760 torr |
| T1 | = | Sampling site temperature (K) |
| T2 | = | 293 K |
| P1 | = | Sampling site pressure (torr) |
| P2 | = | 760 torr |
7.5. Reporting Results to the Industrial Hygienist
For PASSIVE DOSIMETER samples, report results to the industrial hygienist as mg/m3 mercury vapor.
For ACTIVE SAMPLERS, report results as:
For mercury vapor result a): If a prefilter was used and the glass wool and sorbent were combined:
mercury vapor = glass wool + sorbent
The prefilter (if used) was present during sampling to assure that mercury particulate was not trapped in the glass wool.
For total mercury result (b): The sum of the mercury found in the sorbent (vapor), glass wool, and prefilter (if used) for each active sampler is considered. This result is used to determine compliance to the Transitional PEL for total mercury. The Transitional PEL considers both the vapor and particulate fractions of mercury.
Any mercury particulate found on the prefilter can be assessed for compliance with the Ceiling PEL for mercury. See reference 8.14. for further details.
If sampling information has not been provided by field personnel, results are reported in total micrograms.
8. References
8.1. SKC Inc.: Gas Monitoring Dosimeter Badge for Mercury (Operating Instructions). Eighty Four, PA: SKC Inc., no publication date given.
8.2. Occupational Safety and Health Administration Technical
Center: Evaluation of Mercury Solid Sorbent Passive
Dosimeter by J. Ku (OSHA-SLTC Backup Report for Method No.
8.3. Moffitt, A.E., Jr. and R.E. Kupel: A Rapid Method Employing Impregnated Charcoal and Atomic Absorption Spectroscopy for the Determination of Mercury. Am. Ind. Hyg. Assoc. J. 32: 614 (1971).
8.4. McCammon, C.S., Jr., S.L. Edwards, R.D. Hull, and W.J.
Woodfin: A Comparison of Four Personal Sampling Methods for the
Determination of Mercury Vapor. Am. Ind. Hyg. Assoc. J.
41:
8.5. Rathje, A.O. and D.H. Marcero: Improved Hopcalite Procedure for the Determination of Mercury Vapor in Air by Flameless Atomic Absorption. Am. Ind. Hyg. Assoc. J. 37: 331 (1976).
8.6. Sax, N.I. and R.J. Lewis Sr., ed.: Hawley's Condensed Chemical Dictionary. 11th ed. New York: Van Nostrand Reirihold Co., 1987.
8.7. Windholz, M., ed.: The Merck Index. 10th ed. Rahway, NJ: Merck & Co. Inc., 1983.
8.8. National Institute for Occupational Safety and Health:
Criteria for a Recommended Standard -- Occupational Exposure to
Inorganic Mercury (DHEW/NIOSH Pub. No.
8.9. Occupational Safety and Health Administration Analytical Laboratory: Detection Limit Study for Mercury Cold Vapor Analysis by C. Merrell. Salt Lake City, UT. 1987 (unpublished).
8.10. Occupational Safety and Health Administration Analytical Laboratory: Quality Control Data - Mercury Cold Vapor Analysis by B. Babcock. Salt Lake City, UT. 1987 (unpublished).
8.11. Occupational Safety and Health Administration Analytical
Laboratory: An Evaluation of Mercury Vapor Sampling Devices
by R. Cee, J. Ku, E. Zimowski, S. Edwards, and J. Septon (OSHA-SLCAL
Product Evaluation No.
8.12. Menke, R. and G. Wallis: Detection of Mercury in Air
in the Presence of Chlorine and Water Vapor. Am. Ind. Hyg. Assoc.
J. 41:
8.13. Occupational Safety and Health Administration Technical Center: An Evaluation of Hopcalite Sampling Methods for Mercury by J. Septon. Salt Lake City, UT. In progress (unpublished).
8.14. Occupational Safety and Health Administrations Technical
Center: Mercury Particualte in Workplace Atmospheres
(OSHA-SLTC Method No.
Cold
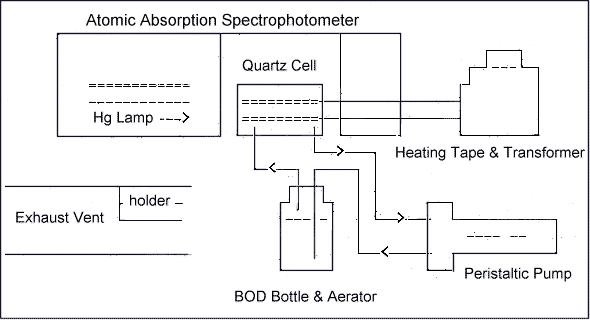
Figure 1